$250 \mathrm{~mL}$ of $0.5 \mathrm{M} \mathrm{NaOH}$ was added to $500 \mathrm{~mL}$ of $1 \mathrm{M}$ $\mathrm{HCl}$. The number of unreacted $\mathrm{HCl}$ molecules in the solution after complete reaction is $\times 10^{21}$. (Nearest integer)
$\left(\mathrm{N}_{\mathrm{A}}=6.022 \times 10^{23}\right)$
We known that no. of moles $=\mathrm{V}_{\text {litre }} \times$ Molarity
\& No. of millimoles $=\mathrm{V}_{\mathrm{ml}} \times$ Molarity
so millimoles of $\mathrm{NaOH}=250 \times 0.5$
$=125$
Millimoles of $\mathrm{HCl}=500 \times 1=500$
Now reaction is
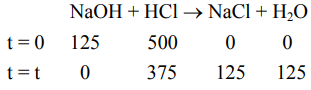
so millimoles of $\mathrm{HCl}$ left $=375$
Moles of $\mathrm{HCl}=375 \times 10^{-3}$
No. of HCl molecules $=6.022 \times 10^{23} \times 375 \times 10^{-3}$
$=225.8 \times 10^{21}$
$\simeq 226 \times 10^{21}=226$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
