Question:
A $5 \%$ solution of cane sugar (molar mass 342 ) is isotonic with $1 \%$ of a solution of an unknown solute. The molar mass of unknown solute in $\mathrm{g} / \mathrm{mol}$ is :-
Correct Option: , 3
Solution:
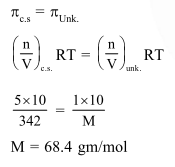
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
