A bakelite beaker has volume capacity of $500 \mathrm{cc}$ at $30^{\circ} \mathrm{C}$. When it is partially filled with $\mathrm{V}_{\mathrm{m}}$ volume (at $30^{\circ}$ ) of mercury, it is found that the unfilled volume of the beaker remains constant as temperature is varied. If $\gamma_{\text {(beaker) }}=6 \times 10^{-6}{ }^{\circ} \mathrm{C}^{-1}$ and $\gamma_{\text {(mercury) }}=1.5 \times 10^{-4}{ }^{\circ} \mathrm{C}^{-1}$, where $\gamma$ is the coefficient of volume expansion, then $\mathrm{V}_{\mathrm{m}}$ (in $\mathrm{cc}$ ) is close to_______.
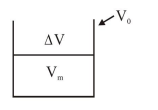
$\Delta \mathrm{V}=\left(\mathrm{V}_{0}-\mathrm{V}_{\mathrm{m}}\right)$
After increasing temperature
$\Delta \mathrm{V}^{\prime}=\left(\mathrm{V}_{0}^{\prime}-\mathrm{V}_{\mathrm{m}}^{\prime}\right)$
$\Delta \mathrm{V}^{\prime}=\Delta \mathrm{V}$
$\mathrm{V}_{0}-\mathrm{V}_{\mathrm{m}}=\mathrm{V}_{0}\left(1+\gamma_{\mathrm{b}} \Delta \mathrm{T}\right)-\mathrm{V}_{\mathrm{m}}\left(1+\gamma_{\mathrm{M}} \Delta \mathrm{T}\right)$
$\mathrm{V}_{0} \gamma_{\mathrm{b}}=\mathrm{V}_{\mathrm{m}} \gamma_{\mathrm{m}}$
$\mathrm{V}_{\mathrm{m}}=\frac{\mathrm{V}_{0} \gamma_{\mathrm{b}}}{\gamma_{\mathrm{m}}}=\frac{(500)\left(6 \times 10^{-6}\right)}{\left(1.5 \times 10^{-4}\right)}$
$=20 \mathrm{CC}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
