Question:
A metal crystallises in a face centred cubic structure. If the edge length of its unit cell is
'a', the closest approach between two atoms in metallic crystal will be :-
Correct Option: , 4
Solution:
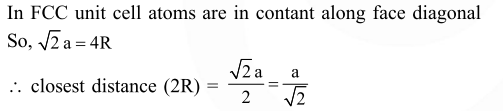
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
