Question:
A solution at $20^{\circ} \mathrm{C}$ is composed of $1.5 \mathrm{~mol}$ of benzene and $3.5 \mathrm{~mol}$ of toluene. If the vapour pressure of pure benzene and pure toluene at this temperature are $74.7$ torr and $22.3$ torr, respectively, then the total vapour pressure of the solution and the benzene mole fraction in equilibrium with it will be, respectively :
Correct Option: 1
Solution:
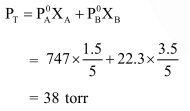
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
