Al2O3 was leached with alkali to get X. The solution of X on passing of gas Y, forms Z. X, Y and Z respectively are :
Question:
$\mathrm{Al}_{2} \mathrm{O}_{3}$ was leached with alkali to get $\mathrm{X}$. The solution of $\mathrm{X}$ on passing of gas $\mathrm{Y}$, forms $\mathrm{Z}$. X, $Y$ and $Z$ respectively are :
Correct Option: , 2
Solution:
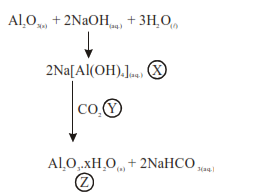
So
$\mathrm{X}: \mathrm{Na}\left[\mathrm{Al}(\mathrm{OH})_{4}\right]$
$\mathrm{Y}: \mathrm{CO}_{2}$
$\mathrm{Z}: \mathrm{Al}_{2} \mathrm{O}_{3} \cdot \mathrm{xH}_{2} \mathrm{O}$
Comments
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
