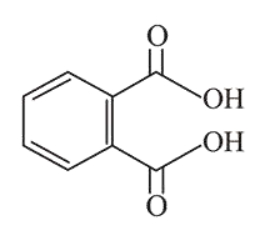
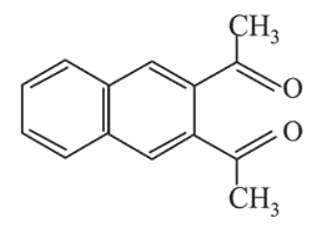
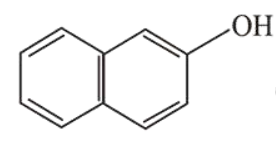
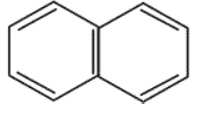
Question:
Among the following four aromatic compounds , which one will have the lowest melting point?
Correct Option: , 4
Solution:
The force of attraction between the molecules affects the melting point of a compound. Polarity increases the intermolecular force of attraction and as a result increases the melting point.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.