Question:
Among the following molecules/ions,
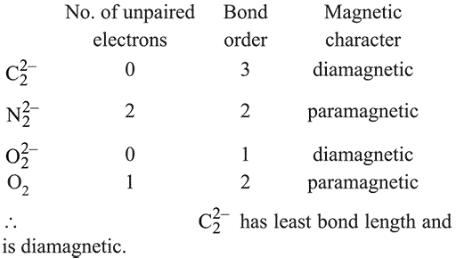
$\mathrm{C}_{2}^{2-}, \mathrm{N}_{2}^{2-}, \mathrm{O}_{2}^{2-}, \mathrm{O}_{2}$
Which one is diamagnetic and has the shortest bond length?
Correct Option: , 4
Solution:
Bond length $\propto \frac{1}{\text { Bond order }}$
and diamagnetic species has no unpaired electron in their molecular orbitals.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
