Question:
An aqueous solution of $\mathrm{NiCl}_{2}$ was heated with excess sodium cyanide in presence of strong oxidizing agent to form $\left[\mathrm{Ni}(\mathrm{CN})_{6}\right]^{2-}$. The total change in number of unpaired electrons on metal centre is
Solution:
$\left[\mathrm{Ni}(\mathrm{CN})_{6}\right]^{2-}$
$\mathrm{Ni}^{+4} \rightarrow \mathrm{d}^{6}$ strong field ligand
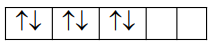
Pairing will be there zero unpaired electron
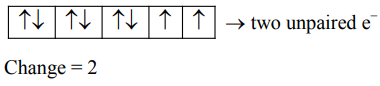
$\mathrm{NiCl}_{2} \rightarrow \mathrm{Ni}^{2+} \rightarrow \mathrm{d}^{8}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
