Assign oxidation numbers to the underlined elements in each of the following species:
(a) $\mathrm{NaH}_{2} \mathrm{PO}_{4}$ (b) $\mathrm{NaHS} \mathrm{SO}_{4}$ (c) $\mathrm{H}_{4} \mathrm{P}_{2} \mathrm{O}_{7}$ (d) $\mathrm{K}_{2} \mathrm{MnO}_{4}$
(e) $\mathrm{Ca} \underline{\mathrm{O}}_{2}$ (f) $\mathrm{NaB} \mathrm{H}_{4}$ (g) $\mathrm{H}_{2} \underline{\mathrm{S}}_{2} \mathrm{O}_{7}$ (h) $\mathrm{KAl}\left(\underline{\mathrm{S}} \mathrm{O}_{4}\right)_{2} \cdot 12 \mathrm{H}_{2} \mathrm{O}$
(a) $\mathrm{NaH}_{2} \underline{\mathrm{PO}}_{4}$
Let the oxidation number of $P$ be $x$.
We know that,
Oxidation number of $\mathrm{Na}=+1$
Oxidation number of $H=+1$
Oxidation number of $O=-2$
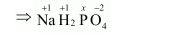
Then, we have
$1(+1)+2(+1)+1(x)+4(-2)=0$
$\Rightarrow 1+2+x-8=0$
$\Rightarrow x=+5$
Hence, the oxidation number of $P$ is $+5$.
(b) $\mathrm{NaHSO}_{4}$
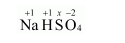
Then, we have
$1(+1)+1(+1)+1(x)+4(-2)=0$
$\Rightarrow 1+1+x-8=0$
$\Rightarrow x=+6$
Hence, the oxidation number of $S$ is $+6$.
(c) $\mathrm{H}_{4} \mathrm{P}_{2} \mathrm{O}_{7}$
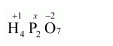
Then, we have
$4(+1)+2(x)+7(-2)=0$
$\Rightarrow 4+2 x-14=0$
$\Rightarrow 2 x=+10$
$\Rightarrow x=+5$
Hence, the oxidation number of $P$ is $+5$.
(d) $\mathrm{K}_{2} \underline{\mathrm{MnO}}_{4}$
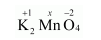
Then, we have
$2(+1)+x+4(-2)=0$
$\Rightarrow 2+x-8=0$
$\Rightarrow x=+6$
Hence, the oxidation number of $\mathrm{Mn}$ is $+6$.
(e) $\mathrm{CaO}_{2}$

Then, we have
$(+2)+2(x)=0$
$\Rightarrow 2+2 x=0$
$\Rightarrow x=-1$
Hence, the oxidation number of $\mathrm{O}$ is $-1$.
(f) $\mathrm{Na} \underline{\mathrm{B}} \mathrm{H}_{4}$
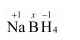
Then, we have
$1(+1)+1(x)+4(-1)=0$
$\Rightarrow 1+x-4=0$
$\Rightarrow x=+3$
Hence, the oxidation number of $B$ is $+3$.
(g) $\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{7}$
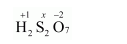
Then, we have
$2(+1)+2(x)+7(-2)=0$
$\Rightarrow 2+2 x-14=0$
$\Rightarrow 2 x=12$
$\Rightarrow x=+6$
Hence, the oxidation number of $S$ is $+6$.
(h) $\mathrm{KAl}\left(\underline{\mathrm{S} \mathrm{O}}_{4}\right)_{2} \cdot 12 \mathrm{H}_{2} \mathrm{O}$
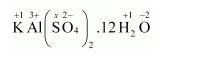
Then, we have
$1(+1)+1(+3)+2(x)+8(-2)+24(+1)+12(-2)=0$
$\Rightarrow 1+3+2 x-16+24-24=0$
$\Rightarrow 2 x=12$
$\Rightarrow x=+6$
Or,
We can ignore the water molecule as it is a neutral molecule. Then, the sum of the oxidation numbers of all atoms of the water molecule may be taken as zero. Therefore, after ignoring the water molecule, we have
$1(+1)+1(+3)+2(x)+8(-2)=0$
$\Rightarrow 1+3+2 x-16=0$
$\Rightarrow 2 x=12$
$\Rightarrow x=+6$
Hence, the oxidation number of $S$ is $+6$.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
