Question.
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 (assume the density of water to be one)
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 (assume the density of water to be one)
Solution:
Mole fraction of $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}=\frac{\text { Number of moles of } \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}}{\text { Number of moles of solution }}$
$0.040=\frac{n_{\mathrm{C}_{2} \mathrm{H}_{3} \mathrm{OH}}}{n_{\mathrm{C}_{2} \mathrm{H}_{3} \mathrm{OH}}+n_{\mathrm{H}_{2} \mathrm{O}}}$ .....(i)
Number of moles present in 1 L water
$n_{\mathrm{H}_{2} \mathrm{O}}=\frac{1000 \mathrm{~g}}{18 \mathrm{~g} \mathrm{~mol}^{-1}}$
$n_{\mathrm{H}_{2} \mathrm{O}}=55.55 \mathrm{~mol}$
Substituting the value of $n_{\mathrm{H}, \mathrm{O}}$ in equation (1),
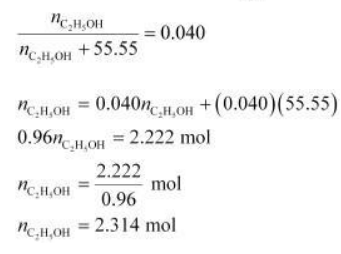
$\therefore$ Molarity of solution $=\frac{2.314 \mathrm{~mol}}{1 \mathrm{~L}}$
$=2.314 \mathrm{M}$
Mole fraction of $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}=\frac{\text { Number of moles of } \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}}{\text { Number of moles of solution }}$
$0.040=\frac{n_{\mathrm{C}_{2} \mathrm{H}_{3} \mathrm{OH}}}{n_{\mathrm{C}_{2} \mathrm{H}_{3} \mathrm{OH}}+n_{\mathrm{H}_{2} \mathrm{O}}}$ .....(i)
Number of moles present in 1 L water
$n_{\mathrm{H}_{2} \mathrm{O}}=\frac{1000 \mathrm{~g}}{18 \mathrm{~g} \mathrm{~mol}^{-1}}$
$n_{\mathrm{H}_{2} \mathrm{O}}=55.55 \mathrm{~mol}$
Substituting the value of $n_{\mathrm{H}, \mathrm{O}}$ in equation (1),
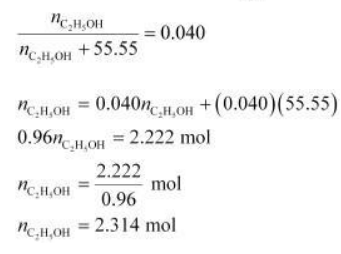
$\therefore$ Molarity of solution $=\frac{2.314 \mathrm{~mol}}{1 \mathrm{~L}}$
$=2.314 \mathrm{M}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
