Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
The given redox reaction can be represented as:
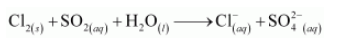
The oxidation half reaction is:
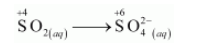
The oxidation number is balanced by adding two electrons as:
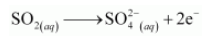
The charge is balanced by adding 4H+ ions as:
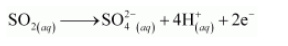
The O atoms and H+ ions are balanced by adding 2H2O molecules as:
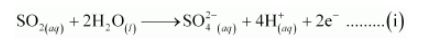
The reduction half reaction is:
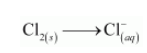
The chlorine atoms are balanced as:
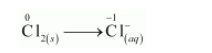
The oxidation number is balanced by adding electrons
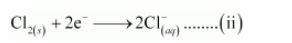
The balanced chemical equation can be obtained by adding equation (i) and (ii) as:
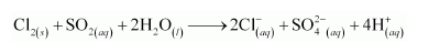
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
