Question:
Complete combustion of $3 \mathrm{~g}$ of ethane gives $\mathrm{x} \times 10^{22}$ molecules of water.
The value of $x$ is ______________ .(Round off to the Nearest Integer).
$\left[\right.$ Use $: \mathrm{N}_{\mathrm{A}}=6.023 \times 10^{23} ;$ Atomic masses in $\mathrm{u}$$: C: 12.0 ; O: 16.0 ; H: 1.0]$
Solution:
(18)
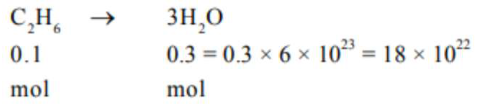
No. of molecules $=0.3 \times 6.023 \times 10^{23}$
$=18.069 \times 10^{22}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
