Question.
Composition of the nuclei of two atomic species X and Y are given as under
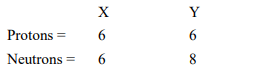
Give the mass numbers of X and Y. What is the relation between the two species?
Composition of the nuclei of two atomic species X and Y are given as under
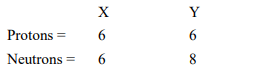
Give the mass numbers of X and Y. What is the relation between the two species?
Solution:
Mass number of X = Number of protons + Number of neutrons = 6 + 6 = 12
Mass number of Y = Number of protons + Number of neutrons = 6 + 8 = 14
These two atomic species X and Y have the same atomic number, but different mass numbers.
Hence, they are isotopes.
Mass number of X = Number of protons + Number of neutrons = 6 + 6 = 12
Mass number of Y = Number of protons + Number of neutrons = 6 + 8 = 14
These two atomic species X and Y have the same atomic number, but different mass numbers.
Hence, they are isotopes.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
