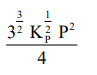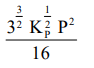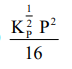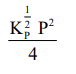Question:
Consider the reaction,
$\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g})$
The equilibrium constant of the above reaction is $\mathrm{K}_{\mathrm{P}}$. If pure ammonia is left to dissociate, the partial pressure of ammonia at equilibrium is given by
(Assume that $\mathrm{P}_{\mathrm{NH}_{3}}<<\mathrm{P}_{\text {total }}$ at equilibrium)
Correct Option: , 2
Solution:
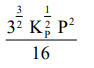
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.