Question:
Consider the van der Waals constants, a and b, for the following gases.

Which gas is expected to have the highest critical temperature?
Correct Option: 1
Solution:
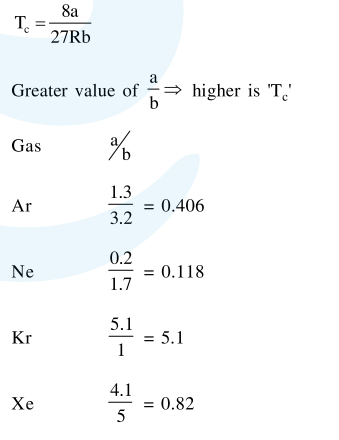
$\therefore \mathrm{T}_{\mathrm{c}}$ has order $: \mathrm{Kr}>\mathrm{Xe}>\mathrm{Ar}>\mathrm{Ne}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
