Question:
Considering the basic strength of amines in aqueous solution, which one has the smallest $\mathrm{pK}_{\mathrm{b}}$ value ?
Correct Option: , 3
Solution:
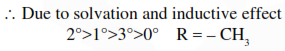
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
