Data given for the following reaction is as follows:
$\mathrm{FeO}_{(\mathrm{s})}+\mathrm{C}_{\text {(graplite) }} \longrightarrow \mathrm{Fe}_{(\mathrm{s})}+\mathrm{CO}_{(\mathrm{g})}$
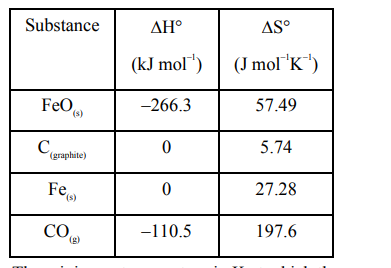
The minimum temperature in K at which the reaction becomes spontaneous is ________ .
(Integer answer)
$\mathrm{T}_{\min }=\left(\frac{\Delta^{0} \mathrm{H}}{\Delta^{0} \mathrm{~S}}\right)$
$\Delta^{0} \mathrm{H}_{\mathrm{rxn}}=\left[\Delta_{\mathrm{f}}^{0} \mathrm{H}(\mathrm{Fe})+\Delta_{\mathrm{f}}^{0} \mathrm{H}(\mathrm{CO})\right]-$
$=\left[\Delta_{\mathrm{f}}^{0} \mathrm{H}(\mathrm{Fe} \mathrm{O})+\Delta_{\mathrm{f}}^{0} \mathrm{H}\left(\mathrm{C}_{\text {(graphite) }}\right)\right]$
$=[0-110.5]-[-266.3+0]$
$=155.8 \mathrm{~kJ} / \mathrm{mol}$
$\Delta^{0} \mathrm{~S}_{\mathrm{rxn}}=\left[\Delta^{0} \mathrm{~S}(\mathrm{Fe})+\Delta^{0} \mathrm{~S}(\mathrm{CO})\right]-$
$\left[\Delta^{0} \mathrm{~S}(\mathrm{Fe} \mathrm{O})+\Delta^{0} \mathrm{~S}\left(\mathrm{C}_{\text {(graphite) }}\right)\right]$
$=[27.28+197.6]-[57.49+5.74]$
$=161.65 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$
$\mathrm{T}_{\min }=\frac{155.8 \times 10^{3} \mathrm{~J} / \mathrm{mol}}{161.65 \mathrm{~J} / \mathrm{mol}-\mathrm{K}}=963.8 \mathrm{~K}$
$\simeq 964 \mathrm{k}$ (nearest integer)
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
