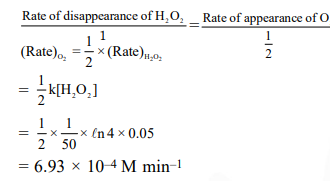
Decomposition of $\mathrm{H}_{2} \mathrm{O}_{2}$ follows a first order reaction. In fifty minutes the concentration of $\mathrm{H}_{2} \mathrm{O}_{2}$ decreases from $0.5$ to $0.125 \mathrm{M}$ in one such decomposition. When the concentration of $\mathrm{H}_{2} \mathrm{O}_{2}$ reaches $0.05 \mathrm{M}$, the rate of formation of $\mathrm{O}_{2}$ will be :-
Correct Option: , 3
$\mathrm{H}_{2} \mathrm{O}_{2(\mathrm{aq})} \longrightarrow \mathrm{H}_{2} \mathrm{O}_{(\mathrm{aq})}+\frac{1}{2} \mathrm{O}_{2}(\mathrm{~g})$
$k=\frac{1}{t} \ln \left(\frac{a_{0}}{a_{1}}\right)$
$=\frac{1}{50} \ln \left(\frac{0.5}{0.125}\right)$
$=\frac{1}{50} \operatorname{\ell n} 4 \min ^{-1}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
