Question:
Describe the shapes of $\mathrm{BF}_{3}$ and $\mathrm{BH}_{4}^{-}$. Assign the hybridisation of boron in these species.
Solution:
(i) BF3
As a result of its small size and high electronegativity, boron tends to form monomeric covalent halides. These halides have a planar triangular geometry. This triangular shape is formed by the overlap of three sp2 hybridised orbitals of boron with the sp orbitals of three halogen atoms. Boron is sp2 hybridised in BF3.
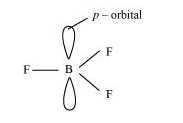
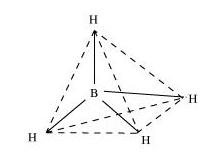
(ii) BH4–
Boron-hydride ion (BH4–) is formed by the sp3 hybridisation of boron orbitals. Therefore, it is tetrahedral in structure.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
