Question:
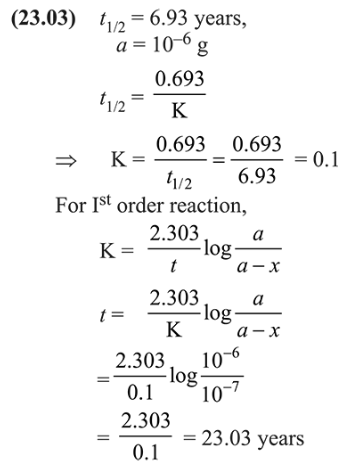
During the nuclear explosion, one of the products is ${ }^{90} \mathrm{Sr}$ with half life of $6.93$ years. If $1 \mu \mathrm{g}$ of ${ }^{90} \mathrm{Sr}$ was absorbed in the bones of a newly born baby in place of $\mathrm{Ca}$, how much time, in years, is require to reduce it by $90 \%$ if it is not lost metabolically ______________ .
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
