Question:
Elements of group 14
(a) exhibit oxidation state of $+4$ only
(b) exhibit oxidation state of $+2$ and $+4$
(c) form $\mathrm{M}^{2-}$ and $\mathrm{M}^{4+}$ ion
(d) form $\mathrm{M}^{2+}$ and $\mathrm{M}^{4+}$ ions
Solution:
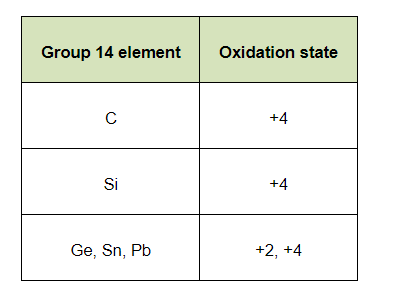
(b) The elements of group 14 have 4 valence electrons. Therefore, the oxidation state of the group is $+4$. However, as a result of the inert pair effect, the lower oxidation state becomes more and more stable and the higher oxidation state becomes less stable. Therefore, this group exhibits $+4$ and $+2$ oxidation states.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
