Question:
Elevation in the boiling point for 1 molal solution of glucose is $2 \mathrm{~K}$. The depression in the freezing point of 2 molal solutions of glucose in the same solvent is $2 \mathrm{~K}$. The relation between $\mathrm{K}_{\mathrm{b}}$ and $\mathrm{K}_{\mathrm{f}}$ is:
Correct Option: , 2
Solution:
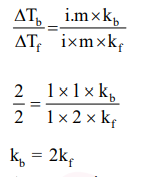
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
