Question:
Explain the important aspects of resonance with reference to the $\mathrm{CO}_{3}^{2-}$ ion.
Solution:
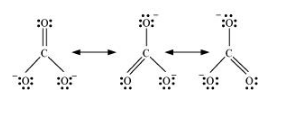
According to experimental findings, all carbon to oxygen bonds in $\mathrm{CO}_{3}^{2-}$ are equivalent. Hence, it is inadequate to represent $\mathrm{CO}_{3}^{2-}$ ion by a single $\mathrm{Lewis}$ structure having two single bonds and one double bond.
Therefore, carbonate ion is described as a resonance hybrid of the following structures:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
