Question.
Explain the mechanism of the cleansing action of soaps.
Explain the mechanism of the cleansing action of soaps.
solution:
A molecule of soap has two dissimilar ends. At one end is the hydrocarbon chain, which is water repellent and the other end is carboxylate anion which is polar end.
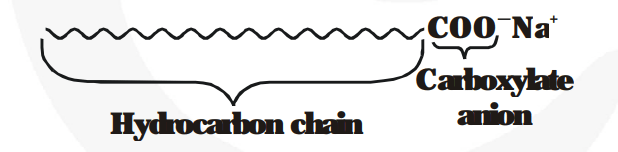
When soap is dissolved in water, many molecules come together and form a group called micelle. These micelles are formed because their hydrocarbon chains come together and the polar ends are projected outward.
When a cloth with a spot of oil is soaked into a soap solution, soap dissolves tiny oil droplets by the hydrophobic end in the middle of the micelle. Due to the outer polar ends, these micelles dissolve in water and are washed away. In this way cloth gets cleaned.
A molecule of soap has two dissimilar ends. At one end is the hydrocarbon chain, which is water repellent and the other end is carboxylate anion which is polar end.

When soap is dissolved in water, many molecules come together and form a group called micelle. These micelles are formed because their hydrocarbon chains come together and the polar ends are projected outward.
When a cloth with a spot of oil is soaked into a soap solution, soap dissolves tiny oil droplets by the hydrophobic end in the middle of the micelle. Due to the outer polar ends, these micelles dissolve in water and are washed away. In this way cloth gets cleaned.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
