Question:
Explain why the following systems are not aromatic?
(i)
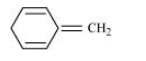
(ii)
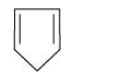
(iii)
Solution:
(i)
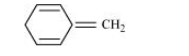
For the given compound, the number of π-electrons is six. But only four π-electrons are present within the ring. Also there is no conjugation of π-electrons within the ring and the compound is not planar in shape. Hence, the given compound is not aromatic in nature.
(ii)

For the given compound, the number of π-electrons is four.
By Huckel’s rule,
$4 n+2=4$
$4 n=2$
$n=\frac{1}{2}$
For a compound to be aromatic, the value of n must be an integer (n = 0, 1, 2…), which is not true for the given compound. Hence, it is not aromatic in nature.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
