Question:
For the decomposition of the compound, represented as
$\mathrm{NH}_{2} \mathrm{COONH}_{4}(\mathrm{~s}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g})+\mathrm{CO}_{2}(\mathrm{~g})$
the $\mathrm{K}_{\mathrm{P}}=2.9 \times 10^{-5} \mathrm{~atm}^{3}$.
If the reaction is started with $1 \mathrm{~mol}$ of the compound, the total pressure at equilibrium would be
Correct Option: , 3
Solution:
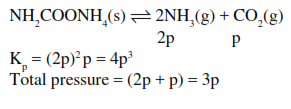
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
