Question:
For the following reactions:
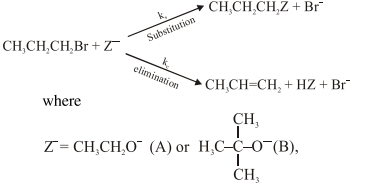
$\mathrm{k}_{\mathrm{s}}$ and $\mathrm{k}_{\mathrm{e}}$, are , respectively, the rate constants for
the substitution and elimination, and $\mu=\frac{\mathrm{k}_{\mathrm{s}}}{\mathrm{k}_{\mathrm{e}}}$, the correct options is -
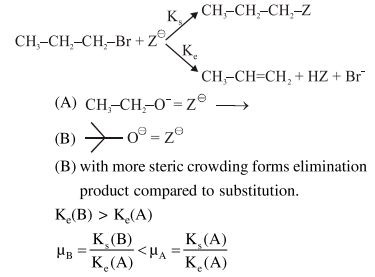
Correct Option: , 3
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
