Question:
For the following reactions
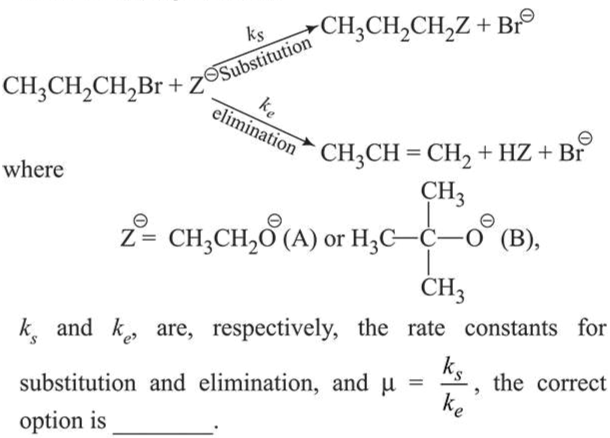
Correct Option: , 2
Solution:
Among the given bases (A) and (B), $t$-butoxide being bulky base favours elimination reaction and ethoxide favours substitution reaction.
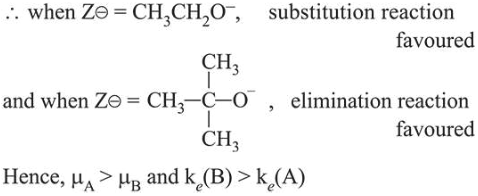
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
