Question:
Given below are two statements:
Statement-I : $\alpha$ and $\beta$ forms of sulphur can change reversibly between themselves with slow heating or slow cooling.
Statement-II : At room temperature the stable crystalline form of sulphur is monoclinic sulphur.
In the light of the above statements, choose the correct answer from the options given below:
Correct Option: , 3
Solution:
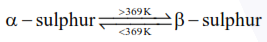
at room temperature $\alpha-$ sulphur (Rhombic) is most stable form.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
