Question.
(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of $\mathrm{Na}_{2} \mathrm{O}$ and $\mathrm{MgO}$ by the transfer of electrons.
(iii) What are the ions present in these compounds?
(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of $\mathrm{Na}_{2} \mathrm{O}$ and $\mathrm{MgO}$ by the transfer of electrons.
(iii) What are the ions present in these compounds?
solution:
(i) The representation of elements with valence electrons as dots around the elements is referred to as electron-dot structure for elements.
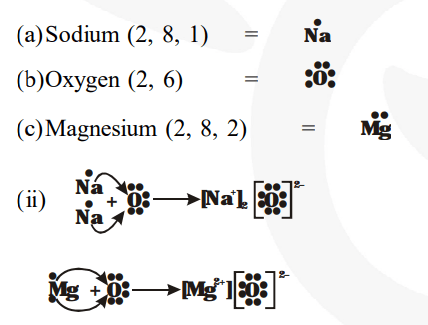
(iii) The ions present in $\mathrm{Na}_{2} \mathrm{O}$ are $\mathrm{Na}^{+}$and $\mathrm{O}^{2-}$ ions and in $\mathrm{MgO}$ are $\mathrm{Mg}^{2+}$ and $\mathrm{O}^{2-}$ ions.
(i) The representation of elements with valence electrons as dots around the elements is referred to as electron-dot structure for elements.
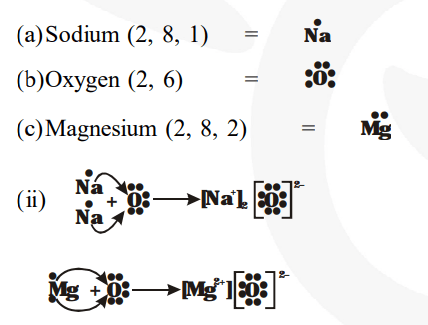
(iii) The ions present in $\mathrm{Na}_{2} \mathrm{O}$ are $\mathrm{Na}^{+}$and $\mathrm{O}^{2-}$ ions and in $\mathrm{MgO}$ are $\mathrm{Mg}^{2+}$ and $\mathrm{O}^{2-}$ ions.
Comments
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
