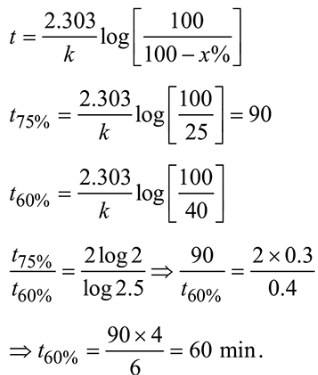
Question:
If $75 \%$ of a first order reaction was completed in 90 minutes, $60 \%$ of the same reaction would be completed in approximately (in minutes) ______________
(Take : $\log 2=0.30 ; \log 2.5=0.40$ )
Solution:
(60)
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
