If minimum possible work is done by a refrigerator in converting 100 grams of water at $0^{\circ} \mathrm{C}$ to ice, how much heat (in calories) is released to the surrounding at temperature $27^{\circ} \mathrm{C}$ (Latent heat of ice $=80 \mathrm{Cal} /$ gram to the nearest integer?
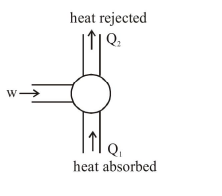
$w+Q_{1}=Q_{2}$
$w=Q_{2}-Q_{1}$
C.O.P. $=\frac{Q_{1}}{w}=\frac{Q_{1}}{Q_{2}-Q_{1}}=\frac{273}{300-273}=\frac{Q_{1}}{W}$
$\mathrm{w}=\frac{27}{273} \times 80 \times 100 \times 4.2$
$\mathrm{Q}_{2}=\mathrm{w}+\theta_{1}$
$\mathrm{Q}_{2}=\frac{27}{273} \times 80 \times 100 \times 4.2+80 \times 100 \times 4.2$
$\mathrm{Q}_{2}=\frac{300}{273} \times 80 \times 100=8791.2 \mathrm{cal}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
