Question:
If the solubility product of $\mathrm{AB}_{2}$ is $3.20 \times 10^{-11} \mathrm{M}^{3}$, then the solubility of $\mathrm{AB}_{2}$ in pure water is__________ $\times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1}$. [Assuming that neither kind of ion reacts with water]
Solution:
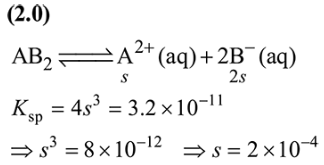
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
