In a pseudo first order hydrolysis of ester in water, the following results were obtained:
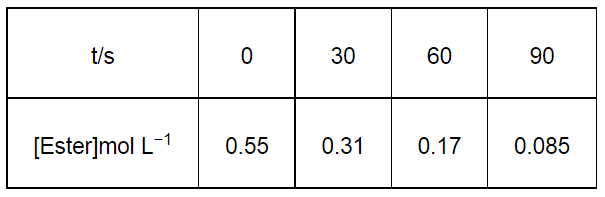
(i) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(ii) Calculate the pseudo first order rate constant for the hydrolysis of ester.
(i) Average rate of reaction between the time interval, 30 to 60 seconds, $=\frac{d[\text { Ester }]}{d t}$
$=\frac{0.31-0.17}{60-30}$
$=\frac{0.14}{30}$
= 4.67 × 10−3 mol L−1 s−1
(ii) For a pseudo first order reaction,
$k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
For $t=30 \mathrm{~s}, k_{1}=\frac{2.303}{30} \log \frac{0.55}{0.31}$
= 1.911 × 10−2 s−1
For $t=60 \mathrm{~s}, k_{2}=\frac{2.303}{60} \log \frac{0.55}{0.17}$
= 1.957 × 10−2 s−1
For $t=90 \mathrm{~s}, k_{3}=\frac{2.303}{90} \log \frac{0.55}{0.085}$
= 2.075 × 10−2 s−1
Then, average rate constant, $k=\frac{k_{1}+k_{2}+k_{3}}{3}$
$=\frac{\left(1.911 \times 10^{-2}\right)+\left(1.957 \times 10^{-2}\right)+\left(2.075 \times 10^{-2}\right)}{3}$
$=1.98 \times 10^{-2} \mathrm{~s}^{-1}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
