Question:
In the button cells widely used in watches and other devices the following reaction takes place:
Zn(s) + Ag2O(s) + H2O(l) → Zn2+(aq) + 2Ag(s) + 2OH−(aq)
Determine $\Delta_{r} G^{\oplus}$ and $E^{\oplus}$ for the reaction.
Solution:
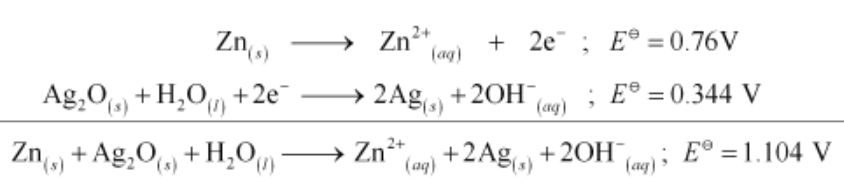
$\therefore E^{\ominus}=1.104 \mathrm{~V}$
We know that,
$\Delta_{r} G^{\ominus}=-n \mathrm{~F} E^{\ominus}$
= −2 × 96487 × 1.04
= −213043.296 J
= −213.04 kJ
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
