Indicate the $\sigma$ and $\pi$ bonds in the following molecules:
$\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{C}_{6} \mathrm{H}_{12}, \mathrm{CH}_{2} \mathrm{Cl}_{2}, \mathrm{CH}_{2}=\mathrm{C}=\mathrm{CH}_{2}, \mathrm{CH}_{3} \mathrm{NO}_{2}, \mathrm{HCONHCH}_{3}$
(i) $\mathrm{C}_{6} \mathrm{H}_{6}$
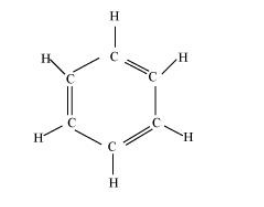
There are six $\mathrm{C}-\mathrm{C}$ sigma $\left(\sigma_{\mathrm{c}-\mathrm{C}}\right)$ bonds, six $\mathrm{C}-\mathrm{H}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{H}}\right)$ bonds, and three $\mathrm{C}=\mathrm{C}$ pi $\left(\pi_{\mathrm{C}-\mathrm{C}}\right)$ resonating bonds in the given compound.
(ii) $\mathrm{C}_{6} \mathrm{H}_{12}$
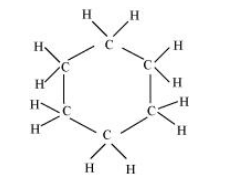
There are six $\mathrm{C}-\mathrm{C}$ sigma $\left(\sigma_{\mathrm{c}-\mathrm{C}}\right)$ bonds and twelve $\mathrm{C}-\mathrm{H}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{H}}\right)$ bonds in the given compound.
(iii) $\mathrm{CH}_{2} \mathrm{Cl}_{2}$
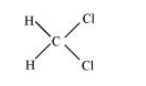
There two $\mathrm{C}-\mathrm{H}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{H}}\right)$ bonds and two $\mathrm{C}-\mathrm{Cl}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{Cl}}\right)$ bonds in the given compound.
(iv) $\mathrm{CH}_{2}=\mathrm{C}=\mathrm{CH}_{2}$
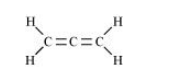
There are two $\mathrm{C}-\mathrm{C}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{C}}\right)$ bonds, four $\mathrm{C}-\mathrm{H}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{H}}\right)$ bonds, and two $\mathrm{C}=\mathrm{C}$ pi $\left(\pi_{\mathrm{C}-\mathrm{C}}\right)$ bonds in the given compound.
(v) $\mathrm{CH}_{3} \mathrm{NO}_{2}$
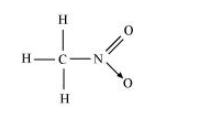
There are three $\mathrm{C}-\mathrm{H}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{H}}\right)$ bonds, one $\mathrm{C}-\mathrm{N}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{N}}\right)$ bond, one $\mathrm{N}-\mathrm{O}$ sigma $\left(\sigma_{\mathrm{N}-0}\right)$ bond, and one $\mathrm{N}=\mathrm{O}$ pi $\left(\pi_{\mathrm{N}-\mathrm{O}}\right)$ bond in the given compound.
(vi) $\mathrm{HCONHCH}_{3}$
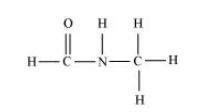
There are two $\mathrm{C}-\mathrm{N}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{N}}\right)$ bonds, four $\mathrm{C}-\mathrm{H}$ sigma $\left(\sigma_{\mathrm{C}-\mathrm{H}}\right)$ bonds, one $\mathrm{N}-\mathrm{H}$ sigma bond, and one $\mathrm{C}=\mathrm{O}$ pi ( $\pi_{\mathrm{C}-\mathrm{C}}$ ) bond in the given compound.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
