Name the members of the lanthanoid series which exhibit +4 oxidation state and those which exhibit +2 oxidation state.
Name the members of the lanthanoid series which exhibit +4 oxidation state and those which exhibit +2 oxidation state. Try to correlate this type of behavior with the electronic configurations of these elements.
The lanthanides that exhibit +2 and +4 states are shown in the given table. The atomic numbers of the elements are given in the parenthesis.
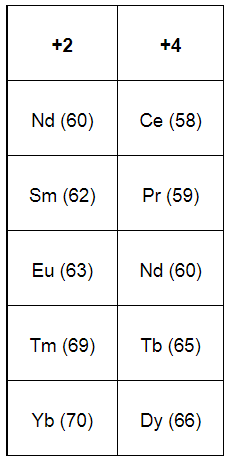
Ce after forming Ce4+ attains a stable electronic configuration of [Xe].
Tb after forming Tb4+ attains a stable electronic configuration of [Xe] 4f7.
Eu after forming Eu2+ attains a stable electronic configuration of [Xe] 4f7.
Yb after forming Yb2+ attains a stable electronic configuration of [Xe] 4f14.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
