Question:
On heating blue coloured powder of copper (II) nitrate, in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed
(a) Write a balanced chemical equation of the reaction.
(b) Identify the brown gas X evolved.
(c) Identify the type of reaction.
(d) What could be the pH range of aqueous solution of the gas X ?
Solution:
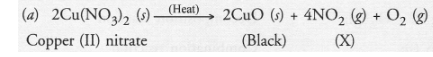
(b) Brown gas (X) is nitrogen dioxide.
(c) It is an example of decomposition reaction.
(d) Nitrogen dioxide is an acidic oxide. Therefore, it dissolves in water to form an acidic solution. The pH of the solution is expected to be less than 7.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
