Question:
Predict if the solutions of the following salts are neutral, acidic or basic:
$\mathrm{NaCl}, \mathrm{KBr}, \mathrm{NaCN}, \mathrm{NH}_{4} \mathrm{NO}_{3}, \mathrm{NaNO}_{2}$ and $\mathrm{KF}$
Solution:
(i) NaCl:
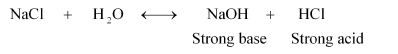
Therefore, it is a neutral solution.
(ii) KBr:

Therefore, it is a neutral solution.
(iii) NaCN:
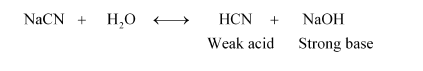
Therefore, it is a basic solution.
(iv) $\mathrm{NH}_{4} \mathrm{NO}_{3}$
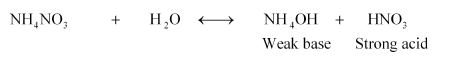
Therefore, it is an acidic solution.
(v) NaNO2
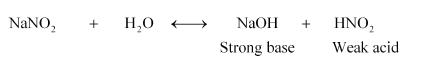
Therefore, it is a basic solution.
(vi) KF
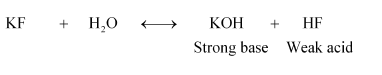
Therefore, it is a basic solution.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
