Question:
Resistance of $0.2 \mathrm{M}$ solution of an electrolyte is $50 \Omega$. The specific conductance of the solution is $1.3 \mathrm{~S} \mathrm{~m}^{-1}$. If resistance of the $0.4 \mathrm{M}$ solution of the same electrolyte is $260 \Omega$, its molar conductivity is :-
Correct Option: , 2
Solution:
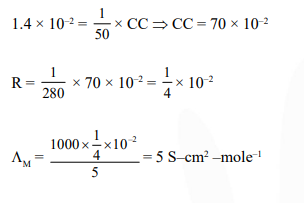
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
