Two salts $A_{2} X$ and $\mathrm{MX}$ have the same value of solubility product of $4.0 \times 10^{-12}$. The ratio of their molar solubilities i.e. $\frac{S\left(A_{2} X\right)}{S(M X)}=$ ______________/(Round off to the Nearest Integer).
(50)
For $A_{2} X$
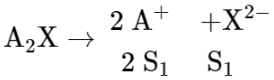
$\mathrm{K}_{\mathrm{sp}}=4 \mathrm{~S}_{1}^{3}=4 \times 10^{-12}$
$\mathrm{S}_{1}=10^{-4}$
for $M X$
$\mathrm{MX} \rightarrow \mathrm{M}^{+}+\mathrm{X}^{-}$
$\mathrm{S}_{2} \quad \mathrm{~S}_{2}$
$\mathrm{K}_{\mathrm{sp}}=\mathrm{S}_{2}^{2}=4 \times 10^{-12}$
$\mathrm{S}_{2}=2 \times 10^{-6}$
So $\frac{\mathrm{S}_{\mathrm{A}_{2} \mathrm{X}}}{\mathrm{S}_{\mathrm{MX}}}=\frac{10^{-4}}{2 \times 10^{-6}}=50$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
