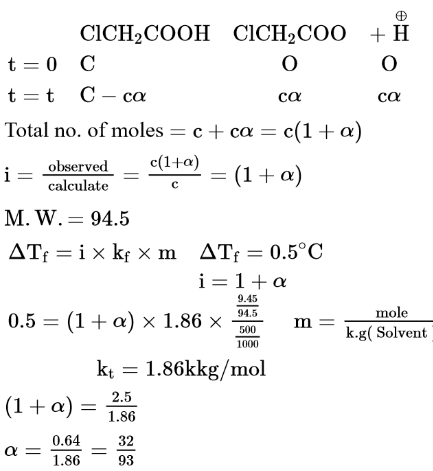
Question:
When $9.45 \mathrm{~g}$ of $\mathrm{ClCH}_{2} \mathrm{COOH}$ is added to $500 \mathrm{~mL}$ of water, its freezing point drops by $0.5^{\circ} \mathrm{C}$. The dissociation constant of $\mathrm{ClCH}_{2} \mathrm{COOH}$ is $\mathrm{x} \times 10^{-3}$. The value of $\mathrm{x}$ is _____________(Rounded off to the nearest integer)
$\left[K_{f\left(H_{2} O\right)}=1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\right]$
Solution:
(35)
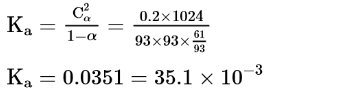
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
