Question:
At $300 \mathrm{~K}$ and 1 atmospheric pressure, $10 \mathrm{~mL}$ of a hydrocarbon required $55 \mathrm{~mL}$ of $\mathrm{O}_{2}$ for complete combustion and $40 \mathrm{~mL}$ of $\mathrm{CO}_{2}$ is formed. The formula of the hydrocarbon is:
Correct Option: , 4
Solution:
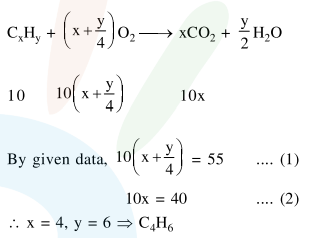
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
