Question:
Spin only magnetic moment in BM of
$\left[\mathrm{Fe}(\mathrm{CO})_{4}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)\right]^{+}$is :
Correct Option: , 4
Solution:
$\left[\mathrm{Fe}(\mathrm{CO})_{4}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)\right]^{+}$
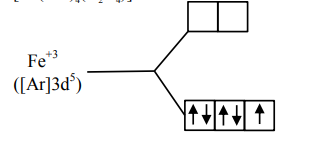
One unpaired electron
Spin only magnetic moment
$=\sqrt{3} \mathrm{~B} \cdot \mathrm{M} .=1.73 \mathrm{BM}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
