Question:
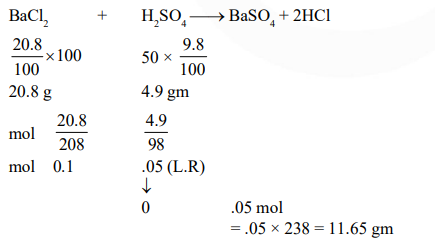
The amount of $\mathrm{BaSO}_{4}$ formed upon mixing $100 \mathrm{~mL}$ of $20.8 \% \mathrm{BaCl}_{2}$ solution with $50 \mathrm{~mL}$ of $9.8 \% \mathrm{H}_{2} \mathrm{SO}_{4}$ solution will be : $(\mathrm{Ba}=137, \mathrm{Cl}=35.5, \mathrm{~S}=32, \mathrm{H}=1$ and $\mathrm{O}=16)$
Correct Option: , 4
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
