Question:
The equilibrium constant for the reaction
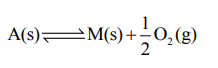
is $\mathrm{K}_{\mathrm{p}}=4$. At equilibrium, the partial pressure of $\mathrm{O}_{2}$ is______ atm. (Round off to the nearest integer)
Solution:
$\mathrm{k}_{\mathrm{p}}=\mathrm{Po}_{2}^{1 / 2}=4$
$\therefore \mathrm{Po}_{2}=16 \mathrm{bar}=16 \mathrm{~atm}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
