The first ionization constant of $\mathrm{H}_{2} \mathrm{~S}$ is $9.1 \times 10^{-8}$. Calculate the concentration of $\mathrm{HS}^{-}$ion in its $0.1 \mathrm{M}$ solution. How will this concentration be affected if the solution is $0.1 \mathrm{M}$ in $\mathrm{HCl}$ also? If the second dissociation constant of $\mathrm{H}_{2} \mathrm{~S}$ is $1.2 \times 10^{-13}$, calculate the concentration of $\mathrm{S}^{2-}$ under both conditions.
(i) To calculate the concentration of HS– ion:
Case I (in the absence of HCl):
Let the concentration of HS– be x M.
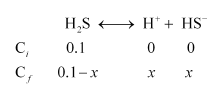
Then, $K_{a_{1}}=\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{HS}^{-}\right]}{\left[\mathrm{H}_{2} \mathrm{~S}\right]}$
$9.1 \times 10^{-8}=\frac{(x)(x)}{0.1-x}$
$\left(9.1 \times 10^{-8}\right)(0.1-x)=x^{2}$
Taking $0.1-x \mathrm{M} ; 0.1 \mathrm{M}$, we have $\left(9.1 \times 10^{-8}\right)(0.1)=x^{2}$.
$9.1 \times 10^{-9}=x^{2}$
$x=\sqrt{9.1 \times 10^{-9}}$
$=9.54 \times 10^{-5} \mathrm{M}$
$\Rightarrow\left[\mathrm{HS}^{-}\right]=9.54 \times 10^{-5} \mathrm{M}$
Case II (in the presence of HCl):
In the presence of $0.1 \mathrm{M}$ of $\mathrm{HCl}$, let $\left[\mathrm{HS}^{-}\right]$be $y \mathrm{M}$.
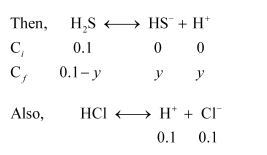
Now, $K_{a_{1}}=\frac{\left[\mathrm{HS}^{-}\right]\left[\mathrm{H}^{+}\right]}{\left[\mathrm{H}_{2} \mathrm{~S}\right]}$
$K_{a_{1}}=\frac{[y](0.1+y)}{(0.1-y)}$
$9.1 \times 10^{-8}=\frac{y \times 0.1}{0.1}$ $(\because 0.1-y ; 0.1 \mathrm{M})$
$9.1 \times 10^{-8}=y$ (and $0.1+y ; 0.1 \mathrm{M}$ )
$\Rightarrow\left[\mathrm{HS}^{-}\right]=9.1 \times 10^{-8}$
(ii) To calculate the concentration of $\left[\mathrm{S}^{2-}\right]$ :
Case I (in the absence of 0.1 M HCl):
$\mathrm{HS}^{-} \longleftrightarrow \mathrm{H}^{+}+\mathrm{S}^{2-}$
$\left[\mathrm{HS}^{-}\right]=9.54 \times 10^{-5} \mathrm{M}$ (From first ionization, case $\mathrm{I}$ )
Let $\left[\mathrm{S}^{2-}\right]$ be $X$.
Also, $\left[\mathrm{H}^{+}\right]=9.54 \times 10^{-5} \mathrm{M}$ (From first ionization, case $\mathrm{l}$ )
$K_{a_{2}}=\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{S}^{2-}\right]}{\left[\mathrm{HS}^{-}\right]}$
$K_{a_{2}}=\frac{\left(9.54 \times 10^{-5}\right)(X)}{9.54 \times 10^{-5}}$
$1.2 \times 10^{-13}=X=\left[\mathrm{S}^{2-}\right]$
Case II (in the presence of 0.1 M HCl):
Again, let the concentration of $\mathrm{HS}^{-}$be $X^{\prime} \mathrm{M}$.
$\left[\mathrm{HS}^{-}\right]=9.1 \times 10^{-8} \mathrm{M}$ (From first ionization, case II)
$\left[\mathrm{H}^{+}\right]=0.1 \mathrm{M}($ From $\mathrm{HCl}$, case $\|$ )
$\left[\mathrm{S}^{2-}\right]=X^{\prime}$
Then, $K_{a_{2}}=\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{S}^{2-}\right]}{\left[\mathrm{HS}^{-}\right]}$
$1.2 \times 10^{-13}=\frac{(0.1)\left(X^{\prime}\right)}{9.1 \times 10^{-8}}$
$10.92 \times 10^{-21}=0.1 X^{\prime}$
$\frac{10.92 \times 10^{-21}}{0.1}=X^{\prime}$
$X^{\prime}=\frac{1.092 \times 10^{-20}}{0.1}$
$=1.092 \times 10^{-19} \mathrm{M}$
$\Rightarrow K_{a_{1}}=1.74 \times 10^{-5}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
