Question:
The half-life of ${ }^{108} \mathrm{Au}$ is $2.7$ days. (a) Find the activity of a sample containing $1.00 \mu \mathrm{g}$ of ${ }^{198} \mathrm{Au}$. (b) What will be the activity after 7 days? Take the atomic weight of ${ }^{198}$ Au to be $198 \mathrm{~g} / \mathrm{mol}$.
Solution:
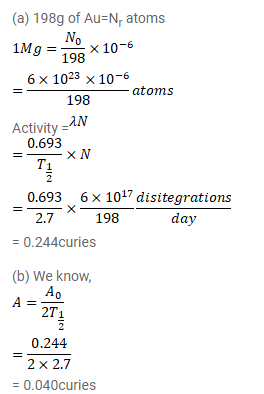
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
