Question:
The ionic radii of $\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}^{+}$and $\mathrm{Mg}^{2+}$ are in the order:
Correct Option: , 2
Solution:
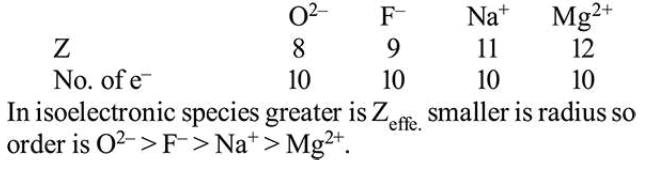
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
